This information is intended for US healthcare professionals. Please confirm you are a healthcare professional to continue.
How should I inject KINERET?
Learn from your healthcare team and develop a routine.
Whether this is your first time injecting or you have used different injection therapies, every treatment is different and we recommend you take advantage of KINERET injection training resources.
The nurses at your doctor’s office may train you to inject or you can receive injection training at any time from the trained nurses at Kineret ON TRACK®.
What happens if I have an injection site reaction?
Most injection site reactions are temporary, and there are tips to help address them.
- Skin reactions are the most common side effect with KINERET and may appear as redness, swelling, bruising, itching, or stinging of the skin at the injection site
- Injection site skin reactions are most common during the first month of treatment and usually last about 14 to 28 days
To help address injection site reactions:
- Cool the site with a cold compress or ice pack for a few minutes, both before and after the injection
- Don't skip the warm-up step of bringing KINERET to room temperature
- Apply hydrocortisone or an antihistamine cream to your injection site
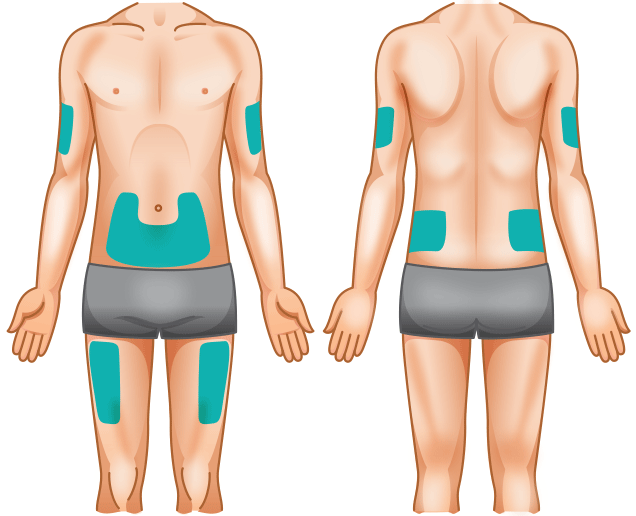
- Rotate sites to avoid soreness. A diary or the KINERET Injection Tracker can help keep track of sites
- Don't inject into skin that is red, bruised, tender, or hard
How do I get KINERET?
Kineret ON TRACK® will arrange for KINERET to be shipped directly to your home.
KINERET is supplied in single-use, prefilled, graduated glass syringes containing 100 mg of KINERET solution. KINERET is dispensed in a 7-day pack and most people receive their KINERET in a shipment of 4 packs (containing a total of 28 injections).

How should I store KINERET?
KINERET should be stored in a refrigerator.
KINERET should be refrigerated at between 36°F to 46°F (2°C to 8°C).
Store KINERET in its original carton and away from light.
Discard any syringe that has been left out of the refrigerator for more than 12 hours.*
Do not freeze or shake KINERET.
*We recommend you take your KINERET syringe out of the refrigerator to allow it to warm to room temperature before injection.
What injection resources are available?
The following resources contain information about injection training.
As a complement to the initial injection instructions you may receive from your doctor’s office, these KINERET training materials contain information that can help you get started.
If you would like to request in-home injection training or get advice via telephone from a KINERET Trained Nurse, Kineret ON TRACK® may be able to help.
KINERET® (anakinra) Injection Tracker
A printable sheet for keeping track of your injection sites
KINERET Treatment Guide
A brochure with information about how KINERET works, how to store and inject KINERET, and details about the services of Kineret ON TRACK®
KINERET Instructions for Use
Instructions for storing and injecting KINERET

If you would like to request injection training or get advice via telephone or video conference from a KINERET Trained Nurse, Kineret ON TRACK® may be able to help.
Learn more about the services available for eligible patients by visiting Kineret ON TRACK®